ArtPlac: developing an ARTificial PLACenta to replace invasive techniques in the treatment of critically ill newborns
Overview
The ArtPlac project was initiated by the European Innovation Council and SMEs Executive Agency (EISMEA) and is being carried out by 10 research and advocacy institutions from Germany, the Netherlands, Sweden, Ireland, and Canada. The project is led by Klinikum Nürnberg and started in April 2023 with an expected run time of 4 years.
The ArtPlac consortium members are: Klinikum Nürnberg, Universiteit Twente, Kungliga Tekniska Hoegskolan, Deutsches Herzzentrum München, Universiteit Maastricht, Fraunhofer Gesellschaft zur Förderung der angewandten Forschung, Maquet Cardiopulmonary GmbH, Arrotek Medical Limited, McMaster University, and EFCNI.
Background
Globally, around two million newborns die every year1. Many of them, especially babies born preterm, are affected by complications of their lungs and kidneys, often leading to failure of those organs.
Previously, the treatment of these issues was often based upon devices and techniques originally designed to suit adult patients. Most common practices as well as many of the advancements in treatment over the last decades were centred around the down-scaling adjustment of originally adults’ medical devices and techniques to fit the smaller scale of newborns. These practices have proven to be quite invasive and while treating certain issues, they often bring side effects to the already vulnerable newborn, thus potentially causing as much harm as they try to alleviate2-5.
Aims and Objectives
The major objective of this project is the introduction of a less invasive way to treat preterm and critically ill newborns in order to prevent complications of their vital organs. While in utero, some of the baby’s vital bodily functions are performed by the placenta, which acts as kidney, lung, and way of feeding. Due to the fact that the placenta cannot be reconnected after birth, this project aims to supply an artificial alternative. The novel device ‘ArtPlac’ can be connected to the belly button and support a newborn’s lung and kidney activities in a similar way an actual placenta does in utero. Using the umbilical vessels at the belly button, ‘ArtPlac’ is powered entirely by the newborn’s own heartbeat and bodily parameters can be collected much less invasively. This way, the new device does not only deliver essential information to the medical professional and provide kidney and lung support to the yet immature organs, but also lets the newborn heal and grow in a more natural setting. Additionally, previous family integration was widely impossible due to the medical equipment hindering physical contact between patient and parents or caregiver. ArtPlac allows much closer contact, which encourages infant- and family-centred development care.
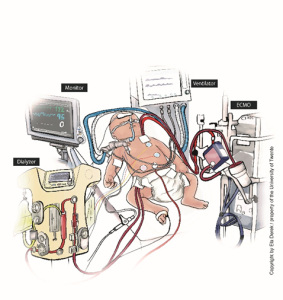
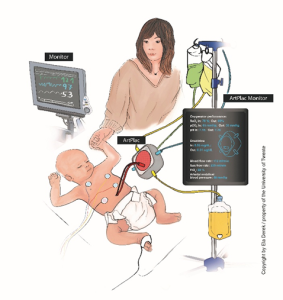
Figure 1A shows a common current treatment situation for newborns, including the many tubes and monitors connected to the baby. 1B depicts the same situation using the new technology in development. The novel device ‘ArtPlac’ is connected to the bellybutton of the baby and provides lung and kidney support as well as monitors essential information of the newborn.
The role of EFCNI
EFCNI is leading the involvement of and cooperation with patients and parents. Our main responsibility is the establishment and subsequent management of a Patient and Consumer Advisory Board (PCAB), in order to ensure the voices of patients and their caregivers are heard. EFCNI provides ethical considerations and includes a more parent and patient-focused perspective in every step of the project. In on-going Steering Committee meetings, EFCNI further offers feedback and advice on the planning and execution of project aims and research goals. Our involvement in the project allows us to raise awareness for these novel processes.
Parent or Caregiver involvement / Patient representation
One major objective of the ArtPlac project is to provide parents and caregivers the opportunity to be close to the newborn during their treatment. Therefore, the involvement of patient and parent representatives is a focal point throughout the entire project, including during the development of the device. Hospital conditions vary greatly and with them the subjective experiences patients and caregivers have. To understand what it is like to live through these moments and how family life as well as future planning is affected, the communication with patient and parent representatives is essential.
The established Patient and Consumer Advisory Board (PCAB), consisting of 5 members from different geographical and professional backgrounds, met with the ArtPlac consortium for a first joint Steering Committee Meeting in January 2024. During this meeting, the individual ArtPlac project teams presented their specific expertise in this endeavour and described current progress, challenges, and findings. Through insightful questions and a fruitful discussion, the PCAB members have brought attention to many points that need to be considered during the design process. Especially the wish to be involved in every stage of the development of such a life-changing medical device was expressed and is welcomed by the ArtPlac team to aid the future progress.
Resources
Official ArtPlac website: Artificial Placenta (ArtPlac) – Preclinical Research Project (under construction)
- Howson, C. P. et al. Reprod Health 10, S1 (2013).
- Heron, M. et al. Natl Vital Stat Rep 57, 1–134 (2009).
- Stoll, B. J. et al. Pediatrics 126, 443–456 (2010).
- Koyner, J. L. Et al. Blood Purif 29, 52–68 (2010).
- Momtaz, H. E. Et al. J Clin Neonatol 3, 99–102 (2014).
Transparency

Acknowledgement of EU Funding: This Project has received funding from the European Union’s Horizon Europe research and innovation programme under the grant agreement No 101099596.
Funded by the European Union. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Union’s Horizon Europe research and innovation programme. Neither the European Union nor the granting authority can be held responsible for them.