When mothers own milk is not available, donor human milk (DHM) is the best alternative to nourish preterm or sick babies. Nevertheless, human milk banks (HMB) within Europe are rarely and heterogeneously regulated. A study reviews these differences in regulations and their implications for the use of DHM. Results confirm the importance of establishing a national and international regulatory framework for HMB.
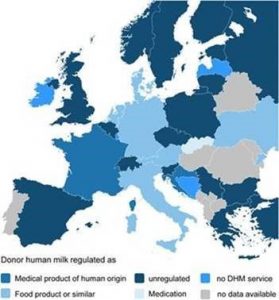
Human milk banks (HMB) serve as facilities to provide preterm or sick babies with donor human milk (DHM), when mothers own milk (MOM) is not available. They also take on the important task of protecting, promoting, and supporting lactation and breastfeeding. According to the European Milk Bank Association (EMBA), there are currently 280 HMBs located in 26 European countries. Unfortunately, in the majority of countries, a legislative framework concerning the use of DHM is lacking, which could discourage the use of DHM or negatively impact the establishment of new HMBs to cover the demand.
Using a cross-sectional survey approved by EMBA, researchers aimed to describe the various regulations of human milk (HM) within 29 European countries, assess its legislative context, and explore the specific impact concerning human milk banking. From June 2020 to February 2021, respondents from 26 of the 29 countries who are involved in HMB and policymaking completed the online questionnaires.
Results show that 9 out of 26 countries define HM as either food product, product of human origin, or medicinal product. In the remainder, DHM remains unclassified. Differences in classification may impede DHM delivery between different jurisdictions (e.g., across borders) and limit the source of supply. The study recognised neonatal care units, tissue and blood banks, and hospital pharmacies as the main operators of HMB. Data concerning the funding of HMB operations were available for ten national services. In those, funding was provided mainly by the operators and, only in three cases, reimbursed by a third party (e.g., Ministries of Health, health insurances). National medical guidelines for the use of DHM were either available or being drafted in 17 countries.
In conclusion, the regulatory framework differs widely within Europe, especially with gaps in the regulation of safety and quality of DHM and protection of donors and recipients. DHM services are unevenly spread, limiting access for vulnerable infants. The development of national medical guidelines is crucial to recognise the provision of human milk as a basic human right and achieve a universal policy in the use of MOM and DHM. These findings could guide stakeholders in establishing a regulatory framework for HMB, thus encouraging this fundamental alternative for preterm or sick babies.
Paper available at: Maternal and Child Nutrition
Full list of authors: Daniel Klotz, Aleksandra Wesołowska, Enrico Bertino, Guido E. Moro, Jean-Charles Picaud, Antoni Gayà, Gillian Weaver.