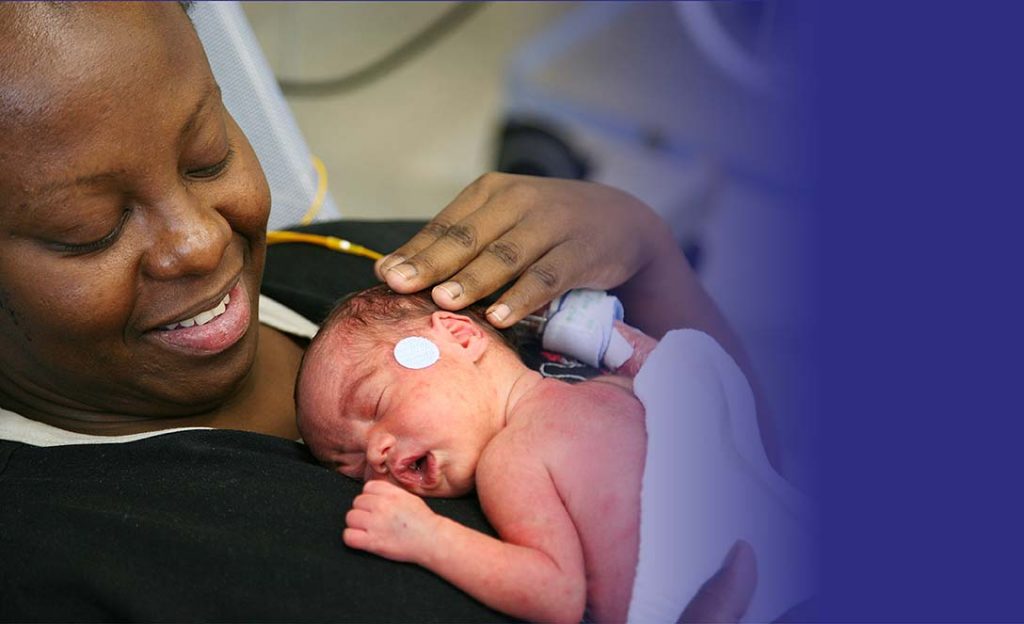
For the best start in life
The European Foundation for the Care of Newborn Infants (EFCNI) is the first pan-European organisation and network to represent the interests of preterm and newborn infants and their families.
We bring together parents, healthcare experts from different disciplines, and scientists with the common goal of improving long-term health of preterm and newborn children. Our vision is to ensure the best start in life for every baby.
With our activities we want to reduce preterm birth rates, ensure the best possible treatment, care, and support and to improve the long-term health of preterm infants and newborns with illnesses.


World Prematurity Day on 17 November is one of the most important days in the year to raise awareness of the challenges and burden of preterm birth globally. Meanwhile, countless individuals and organisations from more than 100 countries join forces with activities, special events and commit to action to help address preterm birth and improve the situation of preterm babies and their families.

World Prematurity Day on 17 November is one of the most important days in the year to raise awareness of the challenges and burden of preterm birth globally. Meanwhile, countless individuals and organisations from more than 100 countries join forces with activities, special events and commit to action to help address preterm birth and improve the situation of preterm babies and their families.
Latest News and Research Updates
Highlighting the power of nutrition: New recommendations on preventing preterm birth through omega-3 fatty acid intake during pregnancy


The European Standards of Care for Newborn Health project is an interdisciplinary collaboration to develop standards of care for key topics in newborn health. The newly developed standards are supported by 108 healthcare professional societies and 50 parent organisations worldwide. The standards were launched in the European Parliament in Brussels on 28 November 2018.

The European Standards of Care for Newborn Health project is an interdisciplinary collaboration to develop standards of care for key topics in newborn health. The newly developed standards are supported by 108 healthcare professional societies and 50 parent organisations worldwide. The standards were launched in the European Parliament in Brussels on 28 November 2018.